Laying the foundation: State-owned roots and technical legacy (1967–2006)
- 1967 The company was originally established as Changsha Zhengzhong Pharmaceutical Machinery Factory; a state-owned enterprise focused on the development and production of pharmaceutical machinery. This early foundation laid the groundwork for decades of technical expertise.
- 1990s Signature products such as the GCB4A liquid filling machine received the National Silver Award, while the domestically developed BXSZ1/20 washing-sterilizing-filling line successfully replaced imported alternatives, reinforcing the company’s strong presence in China’s pharmaceutical equipment sector.
- Pre-2000 With over 30 years of accumulated experience, the company became one of China’s key pharmaceutical machinery exporters, with products reaching markets in Southeast Asia, Eastern Europe, and beyond.
Transformation and expansion: Privatization and global market reach (2006)
- 2006 Following the structural transformation of the original state-owned Changsha Zhengzhong Pharmaceutical Machinery Factory, the company was reestablished as a privately held joint-stock enterprise under the name Hunan Z-Zone Pharmaceutical Machinery Company. The headquarters was relocated to the National High-Tech Industrial Development Zone in Changsha, with a renewed focus on high-end pharmaceutical inspection equipment.
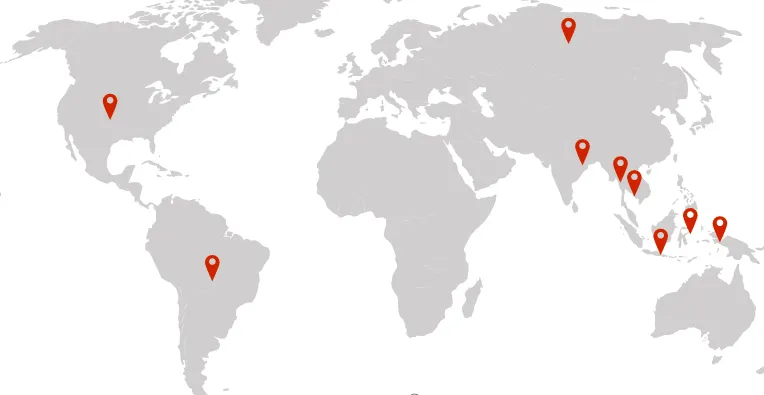
- 2006 milestone In the same year, the company was recognized as the top pharmaceutical machinery company in Chine and launched its internationalization strategy. Our pharmaceutical inspection machinery was exported to 16 countries, including Russia and Brazil, while its customer network expanded to more than 1,500 pharma companies both in China and abroad.
Technological breakthroughs: Filling critical gaps in the domestic market (2008–2013)
-
2008 Z-Zone independently developed the first automatic visual inspection machine for ampoule injection solutions in China. The system passed evaluation by the Hunan Provincial Department of Science and Technology and was recognized for achieving internationally advanced performance standards. This breakthrough ended foreign monopoly in the field and marked a major milestone in domestic substitution.
-
2013 The company introduced the first electronic leak detection machine for ampoule injections in China, reinforcing its technical strength and expanding its presence in the field of intelligent pharmaceutical inspection. Our market share in this specialized segment surpassed 30%.
-
Industry standard leadership Z-Zone led the drafting of two key national industry standards: Automatic Visual Inspection Machine for Ampoule Injection (2011) and Electronic Microleak Detection Machine for Ampoule (2016), strengthening its influence and authority within the pharmaceutical inspection industry.
Global expansion and supply chain advancement (2010s–present)
-
R&D investment
We have established strategic collaborations with institutions such as Central South University and the Chinese Academy of Sciences. The company has formed a dedicated R&D team of over 60 professionals, including PhDs and postgraduates, and built a 12,000 m² research center alongside a 14,000 m² high-standard production facility equipped with more than 100 advanced processing machines.
- Patent portfolio We hold over 200 patents, including 16 invention patents. Our products are ISO 9001:2015 certified and cover a complete portfolio of intelligent inspection equipment for ampoules, vials, and large-volume parenteral.
-
Market growth
Our inspection machines have gained leading market share in emerging regions such as Russia and Southeast Asia, while serving global pharmaceutical clients including Pfizer and Novartis. The company’s current annual production capacity exceeds 150 million RMB.
- Recognition and accreditations We have been recognized as a National High-Tech Enterprise and a National Specialized and Innovative Enterprise, and have remained a preferred supplier of GMP-compliant pharmaceutical inspection equipment to clients in both domestic and international markets.