Since its establishment in 2006, Z-Zone has grown into a specialized high-tech enterprise focused on the development, production, and sale of pharmaceutical inspection equipment. Our solutions are widely used across injectable drug production, biopharmaceuticals, traditional medicine, and food manufacturing. With successful deployments in more than ten countries, we have partnered with pharmaceutical companies worldwide to deliver reliable inspection systems that improve production efficiency, ensure regulatory compliance, and enhance product safety.


In 2024, a pharmaceutical manufacturer in Malaysia approached us with an urgent request for a customized high-precision visual inspection machine, specifically designed to detect impurities in eye drop products. The client placed exceptional emphasis on detection accuracy and consistency.
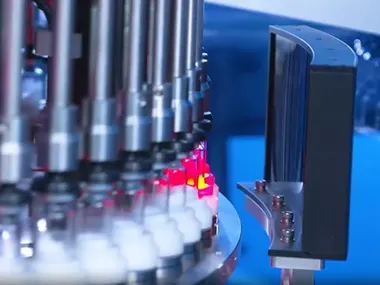
A dedicated team of engineers was assembled immediately to initiate the project. During the early development phase, the team conducted a comprehensive analysis of the client’s eye drop formulation, production process, and quality control benchmarks. Key impurity characteristics such as particle size, type, and shape were thoroughly studied. To meet the performance requirements, the optical inspection system was enhanced with AI capabilities, high-resolution lenses, and optimized lighting configurations. Mechanically, the system was equipped with a high-precision transmission unit and stable positioning structure to ensure accurate handling during the inspection process. Multiple test runs were carried out to fine-tune the mechanics and minimize error margins.
In parallel, the software team developed a highly tailored control program to support fully automated monitoring and operation. This not only increased detection efficiency but also significantly improved accuracy across multiple inspection cycles.
Upon installation, the system delivered outstanding results in detecting microscopic impurities, meeting all expectations. The client expressed strong satisfaction with both the precision and stability of the pharmaceutical inspection machine. This successful implementation demonstrated our team's engineering expertise and laid a solid foundation for future collaboration.