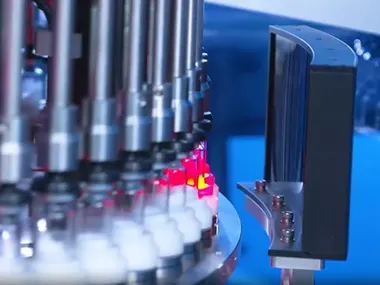
In late 2023, a pharmaceutical company in Brazil, Dechra, placed an order for a fully automatic visual inspection system designed to detect particulate contamination and container defects in ampoules and vials. Given the client's diverse product specifications, a specialized project team was promptly assembled to deliver a tailored solution. Through multiple rounds of in-depth technical discussions, the team gained a comprehensive understanding of the client’s production requirements and quality control standards.
Optimizing the core competencies in visual inspection technology, a fully customized system was developed to meet exacting criteria across various ampoule and vial formats of different specifications. In early 2024, the client traveled to China for a factory acceptance test (FAT). During the on-site demonstration, the system consistently exhibited high accuracy and stability across different ampoule and vial formats. It successfully detected micro-level particulates with precision, earning strong approval from the client’s verification team.
The AVI equipment passed all FAT protocols and speed stability tests with high marks, exceeding the client’s performance expectations. Delivery was completed on schedule in 2024, supported by streamlined production and rigorous quality assurance. This successful collaboration further demonstrated our strength in pharmaceutical inspection systems and reinforced its credibility through responsive service and consistently high product standards. A strong foundation was established for future cooperation.