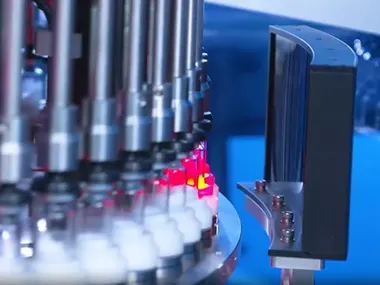
In April 2025, a major milestone was achieved with the successful delivery of a pre-filled syringe (PFS) visual inspection system to B. Braun, a global leader in medical technology headquartered in Germany. This marked a significant step forward in gaining access to the high-standard European market and represented a shift from technology following to independent innovation in pharmaceutical inspection equipment.
The decision by B. Braun, a company with over 180 years of history and a strong reputation for excellence, to implement this inspection system at one of its domestic production sites speaks volumes about the technical capabilities delivered.
Several factors contributed to this breakthrough:- Proven reliability: European regulations demand exceptionally high levels of equipment stability and performance. The ability to meet these expectations confirmed the system’s readiness to compete alongside long-established international brands.
- Market impact: This partnership has created a model case for entering other developed pharmaceutical markets. It also helps shift global perceptions of Chinese manufacturing, highlighting the evolution toward precision engineering and advanced automation.
This collaboration not only validates our technical foundation and global competitiveness but also sets the stage for broader expansion across Europe and North America.