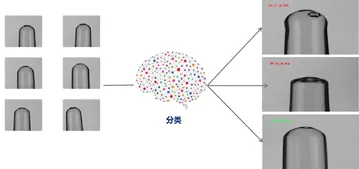
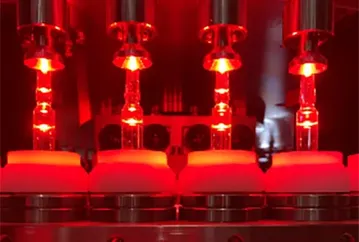
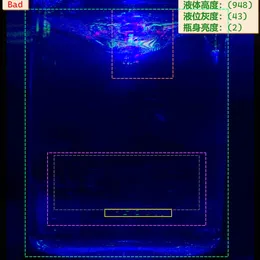
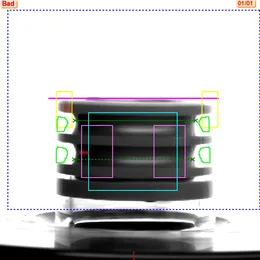
Principles of Visual Image Processing for Pharmaceutical Inspection
Visual image processing detects defects or contaminants in pharmaceutical products, such as injectable solutions, lyophilized powders, and prefilled syringes by analyzing digital images captured by high-resolution cameras or sensors. The process includes several key steps:
Post-Detection Classification
After visual inspection, the extracted features are compared against predefined standards to identify anomalies such as defects on the vial body, contamination, or irregular fill levels. Based on this analysis, unqualified products are rejected and automatically recorded in the backend system.
Advantages
- Non-destructive testing: Inspections are contactless, preserving product integrity.
- High precision: More effective than manual inspection in detecting micron-level defects like cracks, pinholes, and fine particles.
- Speed & scalability: Capable of inspecting thousands of units per minute, making it ideal for high-throughput automated production lines.
- Consistency: Reduces human error and ensures compliance with strict regulatory standards such as GMP.
- Versatility: Algorithms can be customized to inspect a wide range of dosage forms including tablets, liquids, and packaging.
This approach improves the efficiency of quality control, reduces waste, and enhances patient safety throughout the pharmaceutical production process.
Visual Inspection and Regulatory Compliance
Visual inspection, whether manual or automated is a critical step in pharmaceutical production. It ensures both sterility and product appearance, helping to prevent visible particles such as fibers or debris from contaminating the medicine and protecting patient safety.
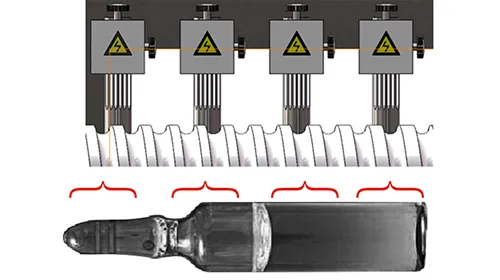
Automated visual inspection uses high-resolution cameras and advanced image processing algorithms to inspect drugs quickly and accurately. It is well-suited for large-scale production and maintains a consistently high standard of inspection, minimizing operator influence.
Sources of Visible Foreign Particles
Visible contaminants are generally categorized as external or internal:
External: Unrelated to the production process, such as hair, non-process-related fibers, starch granules, or mineral dust. These are typically introduced randomly and may carry microbial contamination risks.
Examples of Appearance Defects and Visible Particle Contamination
Regulatory Requirements and Standards
Global pharmaceutical regulations and quality standards have established specific requirements for visual inspection to ensure that each batch of injectable drugs undergoes rigorous quality control. While the details may vary between guidelines, there are many shared expectations across standards such as GMP (Good Manufacturing Practice), FDA (U.S. Food and Drug Administration), and EMA (European Medicines Agency).
- 100% Visual Inspection
All injectable drugs must undergo 100% visual inspection, meaning that every container and its contents in each production batch must be examined to ensure the absence of visible particles and defects. This requirement is designed to minimize the risk of harmful particles or contamination reaching patients.
- Visible Particle Standard
Visible particles are typically defined as those larger than 50 microns in diameter. Regulations stipulate that injectable products must be “essentially free” of visible particles. The United States Pharmacopeia (USP), European Pharmacopeia (EP), and Japanese Pharmacopeia (JP) all mandate this standard. During normal production, any unit found to contain visible particles must be rejected.
These consistent standards help safeguard product quality and protect patients by minimizing the chance of exposure to harmful contaminants.
Since the detection of visible foreign particles is a probabilistic event, it is influenced by factors such as the type of injectable, product formulation, properties of the foreign particles, container material, and the inspector. Therefore, whether using manual or automated visual inspection, it is difficult to achieve 100% detection of visible foreign particles. In addition to eliminating defective products through inspection, it is essential to consider the characteristics of the injectables, emphasize process control, and optimize inspection methods. By identifying and tracing the sources of visible foreign particles, it is possible to prevent contamination at the source and establish a scientific, systematic control framework.